Details of the Drug
General Information of Drug (ID: DMZKGPV)
| Drug Name |
Gemigliptin
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
911637-19-9; UNII-5DHU18M5D6; 5DHU18M5D6; (S)-1-(2-Amino-4-(2,4-bis(trifluoromethyl)-5,6-dihydropyrido[3,4-d]pyrimidin-7(8H)-yl)-4-oxobutyl)-5,5-difluoropiperidin-2-one; (S)-1-(2-amino-4-(2,4-bis(trifluoromethyl)-5,8-dihydropyrido[3,4-d]pyrimidin-7(6H)-yl)-4-oxobutyl)-5,5-difluoropiperidin-2-one; Gemigliptin [INN]; Gemigliptin (prop.INN); SCHEMBL1262740; CHEMBL3707235; CHEBI:134731; ZINC68245464; AKOS025290873; PB11419; DB12412; API0013914; AK170799; 2-Piperidinone, 1-((2S)-2-amino-4-(5,8-dihydro-2,4-
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
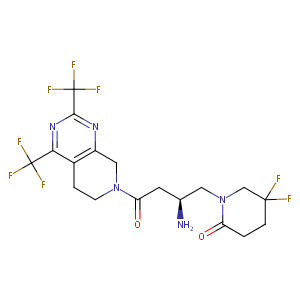 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 489.4 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.7 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 13 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Diabetic complication | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5A2Y | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
